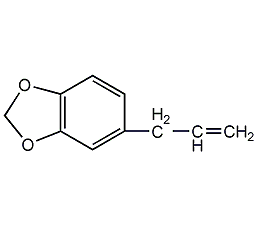
Structural formula
| Business number | 027V |
|---|---|
| Molecular formula | C10H10O2 |
| Molecular weight | 162.19 |
| label |
Allyldioxytolyl, 5-(2-propenyl)-1,3-benzodioxole, 4-allyl-1,2-methylenedioxybenzene, safrole, safrole, 5-(2-propenyl)-3-benzodioxole, 5-Allyl-3-benzodioxole, 4-Allyl-1,2-methylenedioxybenzene, 3,4-Methylenedioxy-allylbenzene, 4-Allylpyrocatechol formaldehyde aceta, soap fragrance |
Numbering system
CAS number:94-59-7
MDL number:MFCD00005841
EINECS number:202-345-4
RTECS number:CY2800000
BRN number:136380
PubChem number:24899851
Physical property data
1. Properties: colorless or slightly yellow liquid with the unique aroma of sassafras tree.
2. Density (g/mL, 20℃): 1.096
3. Phase relative density (20℃, 4℃): 1.1000
4. Melting point (ºC): 11.2
5. Boiling point (ºC, normal pressure): 233 ~235
6. Refractive index at room temperature (n20): 1.5381
7. Refractive index (n20D): 1.537
8. Flash Point (ºC): 100
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 63.8ºC): 1
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol) : Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol /water) logarithmic value of the distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V) : Undetermined
19. Solubility: Easily soluble in alcohol, miscible with chloroform and ether, insoluble in water and glycerol.
Toxicological data
Carcinogenic
Ecological data
None
Molecular structure data
1. Molar refractive index: 46.32
2. Molar volume (cm3/mol): 145.0
3. Isotonic specific volume (90.2K ): 368.0
4. Surface tension (dyne/cm): 41.4
5. Polarizability (10-24cm3): 18.36
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 18.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 167
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Exist in smoke.
2. Naturally found in sassafras oil, camphor oil, nutmeg oil, and cinnamon leaf oil.
3. Easily discolored in strong acid and alkali.
4. It is carcinogenic.
Storage method
Packed in galvanized iron drums or glass bottles. Store in a cool and dry place. Keep away from fire and heat sources
Synthesis method
1. Natural sassafras mainly exists in natural plant essential oils such as sassafras oil, camphor oil, South American Brazilian sassafras oil, and North American sassafras oil. It can be isolated from the above essential oils.
2.Obtained from the isomerization of safrole.
Purpose
1. Safrole can eliminate the smell of grease in soap and is often used to prepare soap flavors.
2. Mainly used for the synthesis of jasmonaldehyde and vanillin.
extended-reading:https://www.newtopchem.com/archives/39787extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyltin-oxide-Ultra-Pure-818-08-6-CAS818-08-6-Dibutyloxotin.pdfextended-reading:https://www.newtopchem.com/archives/44533extended-reading:https://www.cyclohexylamine.net/thermal-catalyst-sa102-polyurethane-thermal-catalyst-sa-102/extended-reading:https://www.newtopchem.com/archives/1112extended-reading:https://www.bdmaee.net/jeffcat-dmp-lupragen-n204-pc-cat-dmp/extended-reading:https://www.cyclohexylamine.net/polyurethane-amine-catalyst-eg-sole-eg-catalyst-eg/extended-reading:https://www.bdmaee.net/polyurethane-catalyst-t-12-cas-77-58-7-niax-d-22/extended-reading:https://www.bdmaee.net/niax-a-1/extended-reading:https://www.morpholine.org/category/morpholine/page/5402/
