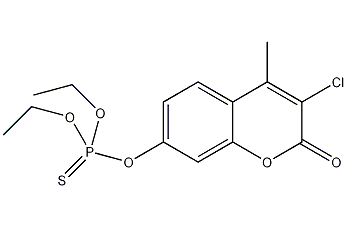
Structural formula
| Business number | 017U |
|---|---|
| Molecular formula | C14H16ClO5PS |
| Molecular weight | 362.77 |
| label |
O-(3-chloro-4-methylcoumarin-7-yl)-O,O-diethylphosphorothioate, coumaphos, Phosphorus, Kuma Fushi, Agridip, Baymix, Coumafos, Meldone, Coumaphoscumafos, O-(3-Chloro-4-methylcoumarin-7-yl)-O,O-diethyl phosphorothioate, Organophosphorus pesticides |
Numbering system
CAS number:56-72-4
MDL number:MFCD00041820
EINECS number:200-285-3
RTECS number:GN6300000
BRN number:327083
PubChem number:24868876
Physical property data
1. Characteristics: Colorless crystal. The industry is brown crystal
2. Density (g/mL,25 /4℃): 1.474
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point ( ºC): 95℃
5. Boiling point ( ºC,Normal pressure): Undetermined
6.p; Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not OK
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturation vapor pressure (kPa,60ºC): Undetermined
13. heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of partition coefficient for water: undetermined
17. Explosion limit (%,V/V): Not OK
18. Lower explosion limit (%,V/V): Not OK
19. Solubility:Slightly soluble in water
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 86.84
2. Molar volume (m3/mol):261.8
3. isotonic specific volume (90.2K):709.5
4. Surface Tension (dyne/cm):53.9
5. Polarizability(10-24cm3): 34.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 86.1
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 500
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be kept sealed.
Synthesis method
(1)200gethyl acetoacetate and270g SO2CI2, respectively dissolved in100mlbenzene, then in10℃, SO2CI2It is obtained by dropping a benzene solution into a benzene solution of ethyl acetoacetate. (2) resorcin88gandα-Ethyl acetoacetate chloride132gDrip640mlIn concentrated sulfuric acid, the temperature is 5Obtained by reaction below ℃3-chlorine-4-Methyl-7-Hydroxycoumarin. 44g 3-Chlorine-4- Methyl-7-Hydroxycoumarin,29gAnhydrous potassium carbonate is placed together500mlIn methyl ethyl ketone, add dropwise under reflux and stirring40g O,O-Diethylphosphorothioate chloride, reflux2h And get fly poison phosphorus.
Purpose
fly poison Phosphorus-based non-systemic insecticides are particularly effective against dipteran pests. They are also used to control external parasites and are effective in controlling skin flies.
extended-reading:https://www.morpholine.org/polyester-sponge-special-catalyst-sponge-catalyst-dabco-ncm/extended-reading:https://www.newtopchem.com/archives/43987extended-reading:https://www.bdmaee.net/n-butyltin-hydroxide-oxide/extended-reading:https://www.bdmaee.net/fascat4201-catalyst-cas-818-08-6-dibutyl-tin-oxide/extended-reading:https://www.newtopchem.com/archives/category/products/page/13extended-reading:https://www.bdmaee.net/low-odor-reaction-type-catalyst/extended-reading:https://www.cyclohexylamine.net/reaction-type-catalyst-9727-polyurethane-amine-catalyst-9727/extended-reading:https://www.bdmaee.net/tertiary-amine-composite-catalyst/extended-reading:https://www.newtopchem.com/archives/39941extended-reading:https://www.newtopchem.com/archives/39958
