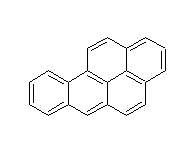
Structural formula
| Business number | 013H |
|---|---|
| Molecular formula | C20H12 |
| Molecular weight | 252.31 |
| label |
Benzo[A]pyrene, Benzo(a)pyrene, 3,4-benzopyrene, Benzopyrene, 1,2-benzopyrene, Benzopyrene standard, Benzo(A)pyrene (standard sample), 3,4-Benzopyrene, Benzo[def]chrysene, 1,2-Benzopyrene, Aromatic hydrocarbons |
Numbering system
CAS number:50-32-8
MDL number:MFCD00003602
EINECS number:200-028-5
RTECS number:DJ3675000
BRN number:1911333
PubChem number:24891610
Physical property data
1. Properties: yellow to brown powder.
2. Density (g/mL, 25/4℃): 1.286
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 177-180
5. Boiling point (ºC, normal pressure): 495
6. Boiling point (ºC, 5.2 kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 495°C
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25 ºC): 0.665×10-19 kPa
12. Saturated vapor pressure (kPa, 60 ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in water , slightly soluble in ethanol, methanol, soluble in benzene, toluene, xylene, chloroform, ether, acetone, etc.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 90.30
2. Molar volume (cm3/mol): 196.0
3. Isotonic specific volume (90.2K ): 553.5
4. Surface tension (dyne/cm): 63.4
5. Polarizability (10-24cm3): 35.80
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 372
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills. The “five pairs” management system for extremely toxic substances should be strictly implemented.
Synthesis method
None
Purpose
1. Histochemical determination of lipids (blue or blue-white fluorescence, fades quickly, cannot be used as a permanent specimen). Cancer research.
2. Often used as a pollution indicator substance for carcinogenic polycyclic aromatic hydrocarbons in the environment.
3. It has no production or use value in industry and is generally only discharged with waste as a by-product formed during the production process.
4. Used to calibrate instruments and devices.
extended-reading:https://www.newtopchem.com/archives/45120extended-reading:https://www.bdmaee.net/dabco-ne210-balance-catalyst-ne210-dabco-amine-catalyst/extended-reading:https://www.bdmaee.net/niax-dmee-low-odor-reactive-catalysts-momentive/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-MP602-delayed-amine-catalyst-non-emission-amine-catalyst.pdfextended-reading:https://www.newtopchem.com/archives/40304extended-reading:https://www.newtopchem.com/archives/category/products/page/159extended-reading:https://www.newtopchem.com/archives/category/products/page/117extended-reading:https://www.bdmaee.net/di-n-butyldichlorotin/extended-reading:https://www.newtopchem.com/archives/40434extended-reading:https://www.newtopchem.com/archives/44242
