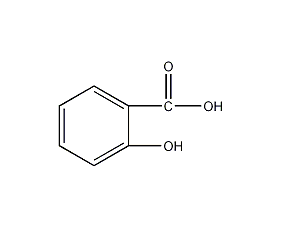
Structural formula
| Business number |
01FS |
| Molecular formula |
C7H6O3 |
| Molecular weight |
138.12 |
| label |
o-hydroxybenzoic acid,
2-hydroxybenzoic acid,
salicylic acid,
Salicic acid,
o-Hydroxybenzoic acid,
2-Hydroxybenzoic acid,
Aromatic carboxylic acids and their derivatives,
acidic solvent
|
Numbering system
CAS number:69-72-7
MDL number:MFCD00002439
EINECS number:200-712-3
RTECS number:VO0525000
BRN number:774890
PubChem number:24899681
Physical property data
1. Properties: White needle-like crystals or monoclinic prisms, with a special phenolic acid smell. It is stable in the air, but gradually changes color when exposed to light.
2. Relative density (g/mL, 20/4℃): 1.443
3. Relative vapor density (g/mL, air=1): 4.8
4. Melting point (ºC): 158~161
5. Boiling point (ºC, 2.67KPa): 210 (2666pa )
6. Relative density (25℃, 4℃): 0.9438159
7. Refractive index(n20D): 1.565
8. Flash point (ºC): 157
9. Gas phase standard heat of combustion (enthalpy) (kJ·mol-1): -3117.2
10. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -494.8
11. Vapor pressure (114ºC): 1mmHg
12. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): 3022.1
13. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -589.9
14. Critical temperature (ºC): No Determine
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17 . Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Slightly soluble in cold water , easily soluble in hot water, ethanol, ether and acetone, soluble in hot benzene. 1 gram of this product can be dissolved in 460 ml of water, 15 ml of hot water, 2.7 ml of alcohol, 3 ml of acetone, 42 ml of chloroform, 3 ml of ether, 135 ml of benzene and 52 ml of turpentine.
Toxicological data
1. LC50 (mouse, intravenous) LC50: 500mg/kg
2. This product irritates the skin and mucous membranes and has a corrosive effect because it can react with proteins in body tissues. . Taking large amounts can cause vomiting, diarrhea, abdominal pain, dyspnea, acidosis and excitement. It can also cause polyuria with polyuria and protein in the urine. Operators should wear labor protection equipment.
Ecological data
This product is slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 35.06
2. Molar volume (cm3/mol): 100.3
3. Isotonic specific volume (90.2K ): 284.4
4. Surface tension (dyne/cm): 64.4
5. Dielectric constant: undetermined
6. Dipole moment (10 -24cm3):
7. Polarizability: 13.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 4
6. Topological molecule polar surface area 57.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable in air. The light gradually changes color. poisonous.
2. It decomposes into phenol and carbon dioxide when heated rapidly under normal pressure. 1g salicylic acid can be dissolved in 460ml water, 15ml boiling water, 2.7ml ethanol, 3ml acetone, 3ml ether, 42ml chloroform, 135ml benzene, 52ml turpentine, about 60ml glycerol and 80ml petroleum ether. Adding sodium phosphate, borax, etc. can increase the solubility of salicylic acid in water. The pH value of aqueous salicylic acid solution is 2.4. Salicylic acid and ferric chloride aqueous solution produce a special purple color.
3. This product irritates the skin and mucous membranes and has a corrosive effect because it can react with proteins in body tissues. Can cause cornea to proliferate and then peel off. It is less toxic than phenol, but taking large amounts can cause vomiting, diarrhea, headache, sweating, rash, shortness of breath, acidosis and excitement. In severe cases, there may be difficulty breathing, collapse, and eventually death from cardiac paralysis. Because salicylic acid is excreted from the kidneys, it often causes acute nephritis. Rabbit oral LD50: 1.3mg/kg. Operators should wear labor protection equipment.
4. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and smoke.
5. Exists in trace amounts in poplar trees.
Storage method
1. It should be stored in a cool, ventilated and dry warehouse, away from fire and heat sources, and stored separately from explosives and oxidants.
2. Packaging is in lined plastic bags, outer sacks or polypropylene woven bags or fiberboard drums.
3. Isolate from explosives and oxidants. Keep away from light.
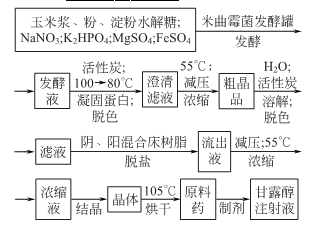
Synthesis method
1. Phenol reacts with sodium hydroxide to generate sodium phenolate. After distillation and dehydration, carbon dioxide is passed through the carboxylation reaction to obtain salicylic acid sodium salt, which is then acidified with sulfuric acid to obtain the crude product. The crude product is refined by sublimation to obtain the finished product. Raw material consumption quota: phenol (98%) 704kg/t, caustic soda (95%) 417kg/t, sulfuric acid (95%) 500kg/t, carbon dioxide (99%) 467kg/t. This method is divided into normal pressure method and medium pressure method. (1) Normal pressure method: Prepare sodium phenolate with 50% sodium hydroxide solution so that the free base is within 1%. After dehydration under reduced pressure, add phenol as a solvent for azeotropic dehydration. Then sodium phenolate is carboxylated by passing dry carbon dioxide into the solvent phenol, and then acidified with sulfuric acid to obtain the finished product.
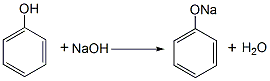

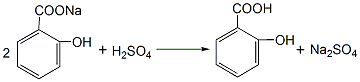
Add phenol and 50% sodium hydroxide into the reactor in a ratio of 1:1.02 (mol). The reaction is dehydrated, and the free base is controlled to ≤1%. After dehydration under reduced pressure, phenol is added as the solvent for azeotropic dehydration. Then, sodium phenolate is introduced into the phenol with dry carbon dioxide gas, and after the carboxylation reaction is performed for 3 hours, carbon dioxide is introduced for the second time for 2 hours, and then the phenol is recovered under reduced pressure, that is, the carboxylation is completed. Then add water to the above sodium salicylate solution to dissolve it into a 50% solution, add 7%-8% sulfuric acid under stirring to acidify to pH 1-2, then cool, filter, and vacuum dry to obtain salicylic acid. The crude product is then sublimated under reduced pressure to obtain the finished product salicylic acid with a content of 99%, with a yield of 50%-70%. (2) Medium pressure method Still using phenol as raw material, sodium phenolate is first made, carboxylated with carbon dioxide under medium pressure to generate phenol carbonate, and then pressurized for molecular rearrangement to generate sodium salicylate, which is then acidified Post-processing produces salicylic acid.  Neutralize the phenol with 50% liquid caustic soda, dry under vacuum, and then cool the kettle temperature to 100℃ , slowly passDry carbon dioxide is added, and when the pressure in the kettle reaches 0.7-0.8Mpa, the flow of carbon dioxide is stopped. At this time, sodium phenol carbonate is generated, and then intramolecular rearrangement isomerization occurs at 130-140°C to become sodium salicylcarboxylate. , and then acidified with sulfuric acid to obtain crude salicylic acid. The crude product is sublimated under reduced pressure to obtain high-quality salicylic acid, with a content of 99% and a yield of more than 98%. Consumption quota (kg/t): phenol (98%) 704, caustic soda (95%) 417, sulfuric acid (95%) 500, carbon dioxide (99%) 467. (3) Preparation method: Phenol reacts with sodium hydroxide to form phenol sodium salt, and then synthesizes sodium o-hydroxybenzoate with carbon dioxide, and then acidifies to produce salicylic acid:
Neutralize the phenol with 50% liquid caustic soda, dry under vacuum, and then cool the kettle temperature to 100℃ , slowly passDry carbon dioxide is added, and when the pressure in the kettle reaches 0.7-0.8Mpa, the flow of carbon dioxide is stopped. At this time, sodium phenol carbonate is generated, and then intramolecular rearrangement isomerization occurs at 130-140°C to become sodium salicylcarboxylate. , and then acidified with sulfuric acid to obtain crude salicylic acid. The crude product is sublimated under reduced pressure to obtain high-quality salicylic acid, with a content of 99% and a yield of more than 98%. Consumption quota (kg/t): phenol (98%) 704, caustic soda (95%) 417, sulfuric acid (95%) 500, carbon dioxide (99%) 467. (3) Preparation method: Phenol reacts with sodium hydroxide to form phenol sodium salt, and then synthesizes sodium o-hydroxybenzoate with carbon dioxide, and then acidifies to produce salicylic acid: The crude product is dissolved in water and decolorized by activated carbon , and then recrystallized and purified. (4) React the same amount of phenol and 50% sodium hydroxide at 105~130℃ to generate phenolSodium, control free base within 1%. After the reaction is completed, dehydrate under reduced pressure, and then use phenol as the solvent to azeotropically dehydrate. After cooling to 100°C, dry carbon dioxide was introduced to carry out carboxylation reaction for 3 hours, and the temperature increased from 128°C to 200°C. After the temperature drops, the solvent phenol is recovered. After 2 to 3 hours, carbon dioxide is introduced for carboxylation for 2 hours, and phenol is recovered under reduced pressure. (Another method of carboxylation is to pass carbon dioxide at 140 to 180°C, and control the reaction pressure to 0.7 to 0.8 MPa for carboxylation reaction for 12 hours.) After the carboxylation reaction is completed, add water to dissolve sodium salicylate and adjust it to 50% solution, add 7% to 8% sulfuric acid solution while stirring to bring the pH value to 1 to 2, cool, filter, spin dry, wash with water several times, spin dry, then heat and dissolve with distilled water, add a little
The crude product is dissolved in water and decolorized by activated carbon , and then recrystallized and purified. (4) React the same amount of phenol and 50% sodium hydroxide at 105~130℃ to generate phenolSodium, control free base within 1%. After the reaction is completed, dehydrate under reduced pressure, and then use phenol as the solvent to azeotropically dehydrate. After cooling to 100°C, dry carbon dioxide was introduced to carry out carboxylation reaction for 3 hours, and the temperature increased from 128°C to 200°C. After the temperature drops, the solvent phenol is recovered. After 2 to 3 hours, carbon dioxide is introduced for carboxylation for 2 hours, and phenol is recovered under reduced pressure. (Another method of carboxylation is to pass carbon dioxide at 140 to 180°C, and control the reaction pressure to 0.7 to 0.8 MPa for carboxylation reaction for 12 hours.) After the carboxylation reaction is completed, add water to dissolve sodium salicylate and adjust it to 50% solution, add 7% to 8% sulfuric acid solution while stirring to bring the pH value to 1 to 2, cool, filter, spin dry, wash with water several times, spin dry, then heat and dissolve with distilled water, add a little
Amount activated carbon, boil, filter while hot, cool the filtrate and dissolve in distilled water again, filter and spin dry several times to obtain pure salicylic acid. The crude salicylic acid obtained can also be refined by sublimation under reduced pressure. The process reaction formula is: 2. Tobacco: OR, 44; OR, 26; FC, 9; FC, 54; BU, 26; FC, 40.
2. Tobacco: OR, 44; OR, 26; FC, 9; FC, 54; BU, 26; FC, 40.
Purpose
1. Mainly used as a raw material in the pharmaceutical industry for the preparation of aspirin, sodium salicylate, salicylamide, analgesic, phenyl salicylate, schistosomiasis-67 and other drugs. In the dye industry, it is used to prepare mordant pure yellow, direct brown 3GN, acid chrome yellow, etc. It is also used as rubber vulcanization retardant and disinfection preservative.
2. Used as an accelerator for epoxy resin curing and as a preservative. It can be used to prepare synthetic fragrances such as methyl salicylate and ethyl salicylate. In the dye industry, it is used as raw material for the preparation of direct dyes and acid dyes. It can also be used as rubber anti-scorch agent, disinfectant, etc.
3. Standards for alkali and iodometric titrations. Fluorescent indicator. Complexation indicator.
4. This product is used as an anti-scorch agent and in the production of ultraviolet absorbers and foaming agents in the rubber industry.
5. Used as fluorescent indicator, matching indicator, and matching masking agent. Developer and preservative for titanium, zirconium, tungsten plasma.
6. Salicylic acid is used as an additive in some weakly acidic electrolytes and can also be used as a complex for electroplating or electroless plating. mixture.
7. Cosmetic preservatives. Mainly used in water cosmetics such as toilet water, prickly heat water, quinine head water, etc. In addition to its antiseptic and sterilizing effects, it also has the functions of removing sweat odor, relieving itching and swelling, and relieving pain and inflammation.
8. A small amount is used to prepare daily flavors such as animal flavor flavors. Used in trace amounts in food as a preservative. It is an important raw material for the pharmaceutical industry.
extended-reading:https://www.newtopchem.com/archives/705extended-reading:https://www.newtopchem.com/archives/1059extended-reading:https://www.bdmaee.net/pc-cat-np10-catalyst-n-dimethylaminopropyldiisopropanolamine/extended-reading:https://www.cyclohexylamine.net/dabco-1027-foaming-retarder/extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Niax-Catalyst-A-1-MSDS.pdfextended-reading:https://www.bdmaee.net/jeffcat-zf-10-catalyst-cas83016-70-0-huntsman/extended-reading:https://www.newtopchem.com/archives/44057extended-reading:https://www.newtopchem.com/archives/850extended-reading:https://www.bdmaee.net/cas-1696-20-4/extended-reading:https://www.newtopchem.com/archives/category/products/page/95







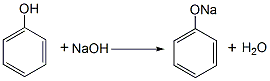

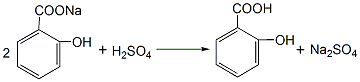
 Neutralize the phenol with 50% liquid caustic soda, dry under vacuum, and then cool the kettle temperature to 100℃ , slowly passDry carbon dioxide is added, and when the pressure in the kettle reaches 0.7-0.8Mpa, the flow of carbon dioxide is stopped. At this time, sodium phenol carbonate is generated, and then intramolecular rearrangement isomerization occurs at 130-140°C to become sodium salicylcarboxylate. , and then acidified with sulfuric acid to obtain crude salicylic acid. The crude product is sublimated under reduced pressure to obtain high-quality salicylic acid, with a content of 99% and a yield of more than 98%. Consumption quota (kg/t): phenol (98%) 704, caustic soda (95%) 417, sulfuric acid (95%) 500, carbon dioxide (99%) 467. (3)
Neutralize the phenol with 50% liquid caustic soda, dry under vacuum, and then cool the kettle temperature to 100℃ , slowly passDry carbon dioxide is added, and when the pressure in the kettle reaches 0.7-0.8Mpa, the flow of carbon dioxide is stopped. At this time, sodium phenol carbonate is generated, and then intramolecular rearrangement isomerization occurs at 130-140°C to become sodium salicylcarboxylate. , and then acidified with sulfuric acid to obtain crude salicylic acid. The crude product is sublimated under reduced pressure to obtain high-quality salicylic acid, with a content of 99% and a yield of more than 98%. Consumption quota (kg/t): phenol (98%) 704, caustic soda (95%) 417, sulfuric acid (95%) 500, carbon dioxide (99%) 467. (3) 
 2. Tobacco: OR, 44; OR, 26; FC, 9; FC, 54; BU, 26; FC, 40.
2. Tobacco: OR, 44; OR, 26; FC, 9; FC, 54; BU, 26; FC, 40.