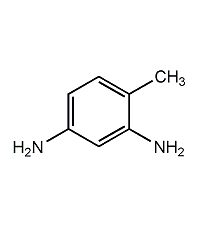
Structural formula
| Business number |
029W |
| Molecular formula |
C7H10N2 |
| Molecular weight |
122.17 |
| label |
4-Methyl-1,3-phenylenediamine,
4-methyl-m-phenylenediamine,
Toluene-2,4-diamine,
m-Toluenediamine,
3-amino-4-methylaniline,
4-methyl-1,3-phenylenediamine,
4-methyl-phenylenediamine,
Hardener,
intermediates,
Aromatic compounds and their derivatives
|
Numbering system
CAS number:95-80-7
MDL number:MFCD00007804
EINECS number:202-453-1
RTECS number:XS9625000
BRN number:2205839
PubChem number:24846558
Physical property data
1.Characteristics: colorless needle-shaped or rhombus crystals. [1]
2. Melting point (℃): 97~99[2]
3. Boiling point (℃) :292[3]
4. Saturated vapor pressure (kPa): 0.13 (106.5℃)[4]
5. Critical pressure (MPa): 4.38[5]
6. Octanol/water partition coefficient: 0.337[6]
7. Ignition temperature (℃): 477[7]
8. Solubility: soluble in water, easily soluble in ethanol, ether, and benzene. [8]
Toxicological data
1. Acute toxicity[9] LD50: 590mg/kg (rat oral, 24h); 650mg/kg (rabbit dermal, 24h)
2. Irritation [10]
Rabbit transdermal: 500mg (24h), mild irritation.
Rabbit eye: 100mg (24h), severe irritation.
3. Mutagenicity [11] Microbial mutagenicity: Salmonella typhimurium 100μg/dish. DNA damage: human fibroblasts 100 μmol/L. Unprogrammed DNA synthesis: human liver 100 μmol/L. Micronucleus test: rats take 300mg/kg orally.
4. Carcinogenicity [12] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
Ecological data
1. Ecotoxicity[13]
LC50: 1420mg/L (96h) (fathead minnow)
EC50: 1290~1440mg/L (96h) (fathead minnow)
2. Biodegradability[14]
Aerobic biodegradation (h): 672~4320
Anaerobic biodegradation (h): 2688~17280
3. Non-biodegradability [15]
Photooxidation half-life in water (h): 31~1740
Photooxidation half-life in air (h): 0.27~2.7
p>
Molecular structure data
1. Molar refractive index: 39.55
2. Molar volume (cm3/mol): 110.2
3. Isotonic specific volume (90.2K ): 296.5
4. Surface tension (dyne/cm): 52.3
5.�Electrical constant:
6. Dipole moment (10-24cm3):
7. Polarizability :15.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 52
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 92.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Toxic. For its toxicity and protection methods, please refer to o-phenylenediamine.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidants, acids, acid chlorides, acid anhydrides, chloroform
4. Conditions to avoid contact [18] Heating
5. Polymerization hazard[19] No polymerization
6. Decomposition products[20 ] Ammonia
Storage method
1. Storage precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Packed in iron drums, 50kg per drum. Keep away from moisture and sun when storing. To prevent oxidation, add an appropriate amount of reducing agent. Store and transport according to regulations on toxic and dangerous goods.
Synthesis method
2,4-dinitrotoluene is obtained by nitration of p-nitrotoluene with mixed acid, and then reduced with iron powder to obtain the crude product, which is then concentrated and distilled to obtain the finished product. The production process is as follows: (1) Nitration  Melted p-nitrotoluene (100%, 643.9kg ) carefully into the nitrification kettle (94.7kg of waste acid is placed in the kettle in advance, and the sulfuric acid content in the waste acid is about 77%. There is no waste acid in the first nitrification, and it can be replaced by sulfuric acid of considerable concentration), stir and cool down the temperature in the kettle to (55±2)℃ and then inject 316.2kg of mixed acid into a thin stream (mixed acid composition: 31.75% nitric acid, 64.85% sulfuric acid, 3.4% water. Dehydration value = 5). In the first stage, half of the mixed acid is added, and the feeding temperature is 52-56°C; in the second stage, half of the mixed acid is added, and the temperature gradually increases from 54°C to 70°C. After the mixed acid is added, the temperature is controlled at (70±2)°C. The time for adding mixed acid depends on the reaction temperature, usually about 6-7 hours. After the mixed acid is added, the temperature is raised to 75°C, kept warm and stirred for 2.25 hours, and the end point is measured. The freezing point reaches above 67°C as qualified. While stirring, carefully and slowly add 70L of cold water for dilution. Control the temperature at 75-77°C. Stir for 15 minutes after adding the water. Stop stirring and let stand for 0.5h, then insert the plastic tube to the bottom of the kettle and cool to room temperature. During the cooling process, please pay attention to condensation. When it is about to condense into a solid, be careful to continuously rotate the plastic tube and avoid muddying the liquid. Wait until all reactants solidify. Pull out the plastic tube, remove the spent acid, and heat to remelt the reactants. The temperature should not exceed 80°C. After melting, stop heating, stir for 3-5 minutes, let stand for 30 minutes, cool, and insert a plastic tube as above. After complete condensation, remove the plastic tube, drain out the waste acid, and reheat to melt. The temperature should not exceed 80°C. The reactant was a yellow oil and was kept at 75°C. Pour 1300L of water into the refining kettle, raise the temperature to 68-70°C, inject the yellow oil in a thin stream under stirring, add liquid caustic soda at any time for neutralization, and strictly control PH=5-6. Keep the temperature of the reactants at 67-68°C, spray cold water to cool down, and yellow needle-like crystals will appear. Continue to stir and cool to about 35°C, filter the material, and wash the filter cake with cold water to obtain yellow crystals of 2. 4-Dinitrotoluene. (2) Reduction
Melted p-nitrotoluene (100%, 643.9kg ) carefully into the nitrification kettle (94.7kg of waste acid is placed in the kettle in advance, and the sulfuric acid content in the waste acid is about 77%. There is no waste acid in the first nitrification, and it can be replaced by sulfuric acid of considerable concentration), stir and cool down the temperature in the kettle to (55±2)℃ and then inject 316.2kg of mixed acid into a thin stream (mixed acid composition: 31.75% nitric acid, 64.85% sulfuric acid, 3.4% water. Dehydration value = 5). In the first stage, half of the mixed acid is added, and the feeding temperature is 52-56°C; in the second stage, half of the mixed acid is added, and the temperature gradually increases from 54°C to 70°C. After the mixed acid is added, the temperature is controlled at (70±2)°C. The time for adding mixed acid depends on the reaction temperature, usually about 6-7 hours. After the mixed acid is added, the temperature is raised to 75°C, kept warm and stirred for 2.25 hours, and the end point is measured. The freezing point reaches above 67°C as qualified. While stirring, carefully and slowly add 70L of cold water for dilution. Control the temperature at 75-77°C. Stir for 15 minutes after adding the water. Stop stirring and let stand for 0.5h, then insert the plastic tube to the bottom of the kettle and cool to room temperature. During the cooling process, please pay attention to condensation. When it is about to condense into a solid, be careful to continuously rotate the plastic tube and avoid muddying the liquid. Wait until all reactants solidify. Pull out the plastic tube, remove the spent acid, and heat to remelt the reactants. The temperature should not exceed 80°C. After melting, stop heating, stir for 3-5 minutes, let stand for 30 minutes, cool, and insert a plastic tube as above. After complete condensation, remove the plastic tube, drain out the waste acid, and reheat to melt. The temperature should not exceed 80°C. The reactant was a yellow oil and was kept at 75°C. Pour 1300L of water into the refining kettle, raise the temperature to 68-70°C, inject the yellow oil in a thin stream under stirring, add liquid caustic soda at any time for neutralization, and strictly control PH=5-6. Keep the temperature of the reactants at 67-68°C, spray cold water to cool down, and yellow needle-like crystals will appear. Continue to stir and cool to about 35°C, filter the material, and wash the filter cake with cold water to obtain yellow crystals of 2. 4-Dinitrotoluene. (2) Reduction  Add 1300L of water to the reduction kettle, and add 500kg of iron powder under stirring. After heating to 70°C, add 40kg of 30% hydrochloric acid, raise the temperature to 90-95°C, take a sample and drop it on the filter paper. If black appears at the intersection of the wetting ring and 5% sulfide alkali solution, you can start adding dinitrogen in batches. Toluene (100% 364kg) makes the reaction proceed vigorously and maintains a boiling state of 100-102°C. When adding materials, check at any time. If a sample is dropped on the filter paper, there will be no yellow residue. The intersection with 5% sulfide alkali solution will appear black, and the intersection with litmus paper should appear red. After adding 75% of the total amount of dinitrotoluene, test the end point. Then add 100kg of iron powder with the same material in a cross-wise manner. The total feeding time is 1-1.5h, and always keep boiling. After adding the material, stir for another 0.5h. The end point is when the reaction solution wets the filter paper and does not show yellow. After the reduction end point is reached, stop heating and cool down to 85-90°C. Carefully add lime slurry made of 30kg of lime and water to make the pH value 8-9. Then add 5kg of 50% alkali sulfide and continue stirring for 15 minutes. The intersection of the wet circle on the filter paper and the 5% sulfide alkali solution without black traces is the end point of neutralization. After neutralization is completed, add 2kg of sodium sulfite, stir for 15 minutes, and let stand for 0.5h to allow the iron mud to fully precipitate. (3) Filtration, concentration and iron sludge sedimentationAfter that, suck the supernatant liquid into the storage tank, add 1000-1200L water to the iron sludge remaining in the reduction kettle, stir and raise the temperature to 95°C and let it stand for 0.5h, then suck the supernatant washing liquid into the storage tank, repeat the above method Wash three times, and the washing liquid is combined into the reduction mother liquor for suction filtration; after suction filtration, the material liquid is evaporated and concentrated under a vacuum of 80kg, which takes about 6 hours to complete. (4) Distillation under reduced pressure: Suction the concentrated liquid into the distillation kettle, and perform dehydration under stirring at a vacuum degree of 74.7-90.7kpa, an interlayer oil temperature of 180-210°C, and a top temperature of 50-60°C. When the vacuum degree rises to 94.7-97.3kpa, the top temperature is 190-200°C, and the interlayer oil temperature is 280-290°C for distillation. As the material in the distillation kettle decreases, the vacuum gradually rises, and the top temperature gradually drops to 170-180°C. ℃, the oil temperature increases to about 300-320 ℃, at which point the distillation can be considered to have reached the end. Each batch of finished products is about 500kg. Raw material consumption quota: p-nitrotoluene 1600kg/t, sulfuric acid (98%) 1616kg/t, nitric acid (98%) 760kg/t, iron powder (90%) 2700kg/t.
Add 1300L of water to the reduction kettle, and add 500kg of iron powder under stirring. After heating to 70°C, add 40kg of 30% hydrochloric acid, raise the temperature to 90-95°C, take a sample and drop it on the filter paper. If black appears at the intersection of the wetting ring and 5% sulfide alkali solution, you can start adding dinitrogen in batches. Toluene (100% 364kg) makes the reaction proceed vigorously and maintains a boiling state of 100-102°C. When adding materials, check at any time. If a sample is dropped on the filter paper, there will be no yellow residue. The intersection with 5% sulfide alkali solution will appear black, and the intersection with litmus paper should appear red. After adding 75% of the total amount of dinitrotoluene, test the end point. Then add 100kg of iron powder with the same material in a cross-wise manner. The total feeding time is 1-1.5h, and always keep boiling. After adding the material, stir for another 0.5h. The end point is when the reaction solution wets the filter paper and does not show yellow. After the reduction end point is reached, stop heating and cool down to 85-90°C. Carefully add lime slurry made of 30kg of lime and water to make the pH value 8-9. Then add 5kg of 50% alkali sulfide and continue stirring for 15 minutes. The intersection of the wet circle on the filter paper and the 5% sulfide alkali solution without black traces is the end point of neutralization. After neutralization is completed, add 2kg of sodium sulfite, stir for 15 minutes, and let stand for 0.5h to allow the iron mud to fully precipitate. (3) Filtration, concentration and iron sludge sedimentationAfter that, suck the supernatant liquid into the storage tank, add 1000-1200L water to the iron sludge remaining in the reduction kettle, stir and raise the temperature to 95°C and let it stand for 0.5h, then suck the supernatant washing liquid into the storage tank, repeat the above method Wash three times, and the washing liquid is combined into the reduction mother liquor for suction filtration; after suction filtration, the material liquid is evaporated and concentrated under a vacuum of 80kg, which takes about 6 hours to complete. (4) Distillation under reduced pressure: Suction the concentrated liquid into the distillation kettle, and perform dehydration under stirring at a vacuum degree of 74.7-90.7kpa, an interlayer oil temperature of 180-210°C, and a top temperature of 50-60°C. When the vacuum degree rises to 94.7-97.3kpa, the top temperature is 190-200°C, and the interlayer oil temperature is 280-290°C for distillation. As the material in the distillation kettle decreases, the vacuum gradually rises, and the top temperature gradually drops to 170-180°C. ℃, the oil temperature increases to about 300-320 ℃, at which point the distillation can be considered to have reached the end. Each batch of finished products is about 500kg. Raw material consumption quota: p-nitrotoluene 1600kg/t, sulfuric acid (98%) 1616kg/t, nitric acid (98%) 760kg/t, iron powder (90%) 2700kg/t. 
Purpose
1. Used as a curing agent for epoxy resin, the reference dosage is 8 parts by mass. The curing conditions are 100℃/2h+150℃/2h, and the thermal change temperature of the cured product is 150-160℃. It is also used as an intermediate for organic synthesis and dyes, such as the manufacture of fur black DB, sulfurized yellow brown 5G, sulfurized red brown BIR and other dyes. Reaction with phosgene can produce 2,4-toluene diisocyanate.
2. It is one of the raw materials for organic synthesis and can be used to prepare toluene diisocyanate. It is also used as a dye intermediate and for hair dyeing. [22]
extended-reading:https://www.bdmaee.net/polyurethane-thermal-delay-catalyst-nt-cate-129-heat-sensitive-metal-catalyst/extended-reading:https://www.newtopchem.com/archives/974extended-reading:https://www.bdmaee.net/u-cat-sa-506-catalyst-cas122987-42-7-sanyo-japan/extended-reading:https://www.bdmaee.net/high-quality-bis3-dimethylaminopropylamino-2-propanol-cas-67151-63-7/extended-reading:https://www.cyclohexylamine.net/k-15-catalyst-potassium-isooctanoate/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-NE1070-polyurethane-gel-type-catalyst–low-odor-catalyst.pdfextended-reading:https://www.newtopchem.com/archives/44038extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/6.jpgextended-reading:https://www.newtopchem.com/archives/44428extended-reading:https://www.bdmaee.net/cas-7560-83-0/





















 Melted p-nitrotoluene (100%, 643.9kg ) carefully into the nitrification kettle (94.7kg of waste acid is placed in the kettle in advance, and the sulfuric acid content in the waste acid is about 77%. There is no waste acid in the first nitrification, and it can be replaced by sulfuric acid of considerable concentration), stir and cool down the temperature in the kettle to (55±2)℃ and then inject 316.2kg of mixed acid into a thin stream (mixed acid composition: 31.75% nitric acid, 64.85% sulfuric acid, 3.4% water. Dehydration value = 5). In the first stage, half of the mixed acid is added, and the feeding temperature is 52-56°C; in the second stage, half of the mixed acid is added, and the temperature gradually increases from 54°C to 70°C. After the mixed acid is added, the temperature is controlled at (70±2)°C. The time for adding mixed acid depends on the reaction temperature, usually about 6-7 hours. After the mixed acid is added, the temperature is raised to 75°C, kept warm and stirred for 2.25 hours, and the end point is measured. The freezing point reaches above 67°C as qualified. While stirring, carefully and slowly add 70L of cold water for dilution. Control the temperature at 75-77°C. Stir for 15 minutes after adding the water. Stop stirring and let stand for 0.5h, then insert the plastic tube to the bottom of the kettle and cool to room temperature. During the cooling process, please pay attention to condensation. When it is about to condense into a solid, be careful to continuously rotate the plastic tube and avoid muddying the liquid. Wait until all reactants solidify. Pull out the plastic tube, remove the spent acid, and heat to remelt the reactants. The temperature should not exceed 80°C. After melting, stop heating, stir for 3-5 minutes, let stand for 30 minutes, cool, and insert a plastic tube as above. After complete condensation, remove the plastic tube, drain out the waste acid, and reheat to melt. The temperature should not exceed 80°C. The reactant was a yellow oil and was kept at 75°C. Pour 1300L of water into the refining kettle, raise the temperature to 68-70°C, inject the yellow oil in a thin stream under stirring, add liquid caustic soda at any time for neutralization, and strictly control PH=5-6. Keep the temperature of the reactants at 67-68°C, spray cold water to cool down, and yellow needle-like crystals will appear. Continue to stir and cool to about 35°C, filter the material, and wash the filter cake with cold water to obtain yellow crystals of 2. 4-Dinitrotoluene. (2) Reduction
Melted p-nitrotoluene (100%, 643.9kg ) carefully into the nitrification kettle (94.7kg of waste acid is placed in the kettle in advance, and the sulfuric acid content in the waste acid is about 77%. There is no waste acid in the first nitrification, and it can be replaced by sulfuric acid of considerable concentration), stir and cool down the temperature in the kettle to (55±2)℃ and then inject 316.2kg of mixed acid into a thin stream (mixed acid composition: 31.75% nitric acid, 64.85% sulfuric acid, 3.4% water. Dehydration value = 5). In the first stage, half of the mixed acid is added, and the feeding temperature is 52-56°C; in the second stage, half of the mixed acid is added, and the temperature gradually increases from 54°C to 70°C. After the mixed acid is added, the temperature is controlled at (70±2)°C. The time for adding mixed acid depends on the reaction temperature, usually about 6-7 hours. After the mixed acid is added, the temperature is raised to 75°C, kept warm and stirred for 2.25 hours, and the end point is measured. The freezing point reaches above 67°C as qualified. While stirring, carefully and slowly add 70L of cold water for dilution. Control the temperature at 75-77°C. Stir for 15 minutes after adding the water. Stop stirring and let stand for 0.5h, then insert the plastic tube to the bottom of the kettle and cool to room temperature. During the cooling process, please pay attention to condensation. When it is about to condense into a solid, be careful to continuously rotate the plastic tube and avoid muddying the liquid. Wait until all reactants solidify. Pull out the plastic tube, remove the spent acid, and heat to remelt the reactants. The temperature should not exceed 80°C. After melting, stop heating, stir for 3-5 minutes, let stand for 30 minutes, cool, and insert a plastic tube as above. After complete condensation, remove the plastic tube, drain out the waste acid, and reheat to melt. The temperature should not exceed 80°C. The reactant was a yellow oil and was kept at 75°C. Pour 1300L of water into the refining kettle, raise the temperature to 68-70°C, inject the yellow oil in a thin stream under stirring, add liquid caustic soda at any time for neutralization, and strictly control PH=5-6. Keep the temperature of the reactants at 67-68°C, spray cold water to cool down, and yellow needle-like crystals will appear. Continue to stir and cool to about 35°C, filter the material, and wash the filter cake with cold water to obtain yellow crystals of 2. 4-Dinitrotoluene. (2) Reduction  Add 1300L of water to the reduction kettle, and add 500kg of iron powder under stirring. After heating to 70°C, add 40kg of 30% hydrochloric acid, raise the temperature to 90-95°C, take a sample and drop it on the filter paper. If black appears at the intersection of the wetting ring and 5% sulfide alkali solution, you can start adding dinitrogen in batches. Toluene (100% 364kg) makes the reaction proceed vigorously and maintains a boiling state of 100-102°C. When adding materials, check at any time. If a sample is dropped on the filter paper, there will be no yellow residue. The intersection with 5% sulfide alkali solution will appear black, and the intersection with litmus paper should appear red. After adding 75% of the total amount of dinitrotoluene, test the end point. Then add 100kg of iron powder with the same material in a cross-wise manner. The total feeding time is 1-1.5h, and always keep boiling. After adding the material, stir for another 0.5h. The end point is when the reaction solution wets the filter paper and does not show yellow. After the reduction end point is reached, stop heating and cool down to 85-90°C. Carefully add lime slurry made of 30kg of lime and water to make the pH value 8-9. Then add 5kg of 50% alkali sulfide and continue stirring for 15 minutes. The intersection of the wet circle on the filter paper and the 5% sulfide alkali solution without black traces is the end point of neutralization. After neutralization is completed, add 2kg of sodium sulfite, stir for 15 minutes, and let stand for 0.5h to allow the iron mud to fully precipitate. (3) Filtration, concentration and iron sludge sedimentationAfter that, suck the supernatant liquid into the storage tank, add 1000-1200L water to the iron sludge remaining in the reduction kettle, stir and raise the temperature to 95°C and let it stand for 0.5h, then suck the supernatant washing liquid into the storage tank, repeat the above method Wash three times, and the washing liquid is combined into the reduction mother liquor for suction filtration; after suction filtration, the material liquid is evaporated and concentrated under a vacuum of 80kg, which takes about 6 hours to complete. (4) Distillation under reduced pressure: Suction the concentrated liquid into the distillation kettle, and perform dehydration under stirring at a vacuum degree of 74.7-90.7kpa, an interlayer oil temperature of 180-210°C, and a top temperature of 50-60°C. When the vacuum degree rises to 94.7-97.3kpa, the top temperature is 190-200°C, and the interlayer oil temperature is 280-290°C for distillation. As the material in the distillation kettle decreases, the vacuum gradually rises, and the top temperature gradually drops to 170-180°C. ℃, the oil temperature increases to about 300-320 ℃, at which point the distillation can be considered to have reached the end. Each batch of finished products is about 500kg. Raw material consumption quota: p-nitrotoluene 1600kg/t, sulfuric acid (98%) 1616kg/t, nitric acid (98%) 760kg/t, iron powder (90%) 2700kg/t.
Add 1300L of water to the reduction kettle, and add 500kg of iron powder under stirring. After heating to 70°C, add 40kg of 30% hydrochloric acid, raise the temperature to 90-95°C, take a sample and drop it on the filter paper. If black appears at the intersection of the wetting ring and 5% sulfide alkali solution, you can start adding dinitrogen in batches. Toluene (100% 364kg) makes the reaction proceed vigorously and maintains a boiling state of 100-102°C. When adding materials, check at any time. If a sample is dropped on the filter paper, there will be no yellow residue. The intersection with 5% sulfide alkali solution will appear black, and the intersection with litmus paper should appear red. After adding 75% of the total amount of dinitrotoluene, test the end point. Then add 100kg of iron powder with the same material in a cross-wise manner. The total feeding time is 1-1.5h, and always keep boiling. After adding the material, stir for another 0.5h. The end point is when the reaction solution wets the filter paper and does not show yellow. After the reduction end point is reached, stop heating and cool down to 85-90°C. Carefully add lime slurry made of 30kg of lime and water to make the pH value 8-9. Then add 5kg of 50% alkali sulfide and continue stirring for 15 minutes. The intersection of the wet circle on the filter paper and the 5% sulfide alkali solution without black traces is the end point of neutralization. After neutralization is completed, add 2kg of sodium sulfite, stir for 15 minutes, and let stand for 0.5h to allow the iron mud to fully precipitate. (3) Filtration, concentration and iron sludge sedimentationAfter that, suck the supernatant liquid into the storage tank, add 1000-1200L water to the iron sludge remaining in the reduction kettle, stir and raise the temperature to 95°C and let it stand for 0.5h, then suck the supernatant washing liquid into the storage tank, repeat the above method Wash three times, and the washing liquid is combined into the reduction mother liquor for suction filtration; after suction filtration, the material liquid is evaporated and concentrated under a vacuum of 80kg, which takes about 6 hours to complete. (4) Distillation under reduced pressure: Suction the concentrated liquid into the distillation kettle, and perform dehydration under stirring at a vacuum degree of 74.7-90.7kpa, an interlayer oil temperature of 180-210°C, and a top temperature of 50-60°C. When the vacuum degree rises to 94.7-97.3kpa, the top temperature is 190-200°C, and the interlayer oil temperature is 280-290°C for distillation. As the material in the distillation kettle decreases, the vacuum gradually rises, and the top temperature gradually drops to 170-180°C. ℃, the oil temperature increases to about 300-320 ℃, at which point the distillation can be considered to have reached the end. Each batch of finished products is about 500kg. Raw material consumption quota: p-nitrotoluene 1600kg/t, sulfuric acid (98%) 1616kg/t, nitric acid (98%) 760kg/t, iron powder (90%) 2700kg/t.