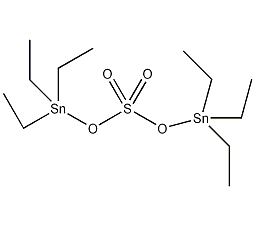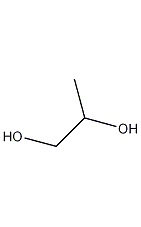
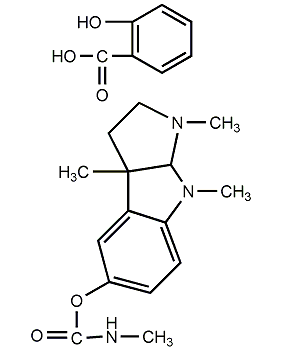
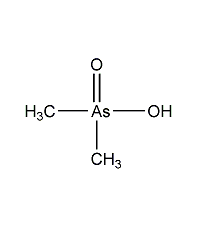
Structural formula
| Business number |
018S |
| Molecular formula |
C3H8O2 |
| Molecular weight |
76.10 |
| label |
propylene glycol,
1,2-dihydroxypropane,
α-propylene glycol,
Methyl glycol,
propylene glycol,
One propyl alcohol,
1,2-Dihydroxypropanol,
Propylene glycol,
1,2-Dihydroxy-propane,
Methyl glycol,
Aliphatic alcohols, ethers and their derivatives
|
Numbering system
CAS number:57-55-6
MDL number:MFCD00064272
EINECS number:200-338-0
RTECS number:TY2000000
BRN number:1340498
PubChem number:24864713
Physical property data
1. Properties: Colorless, viscous and stable water-absorbing liquid, almost tasteless and odorless, flammable, low toxicity.
2. Boiling point (ºC, 101.3kPa): 187.3
3. Melting point (ºC, pouring point): -60
4. Relative density (g /mL, 20/20ºC): 1.0381
5. Relative density (20℃, 4℃): 1.0362
6. Refractive index (n20ºC): 1.4329
7. Viscosity (mPa·s, 0ºC): 243
8. Viscosity (mPa·s, 20ºC): 56.0
9. Viscosity (mPa·s, 40ºC) : 18
10. Flash point (ºC, closed): 98.9
11. Flash point (ºC, open): 107
12. Fire point (ºC ): 421.1
13. Heat of combustion (KJ/mol, constant pressure): 1827.5
14. Heat of combustion (KJ/mol, constant volume): 1825.0
15. Heat of combustion (KJ/mol, 20ºC, 101.3kPa): 1853.1
16. Heat of evaporation (KJ/kg): 538.1
17. Heat of generation (KJ/ mol, 20ºC): 500.3
18. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 2.48
19. Critical temperature (ºC): 351
20. Critical pressure (MPa): 5.9
21. Thermal conductivity (W/(m·K)): 0.217714
22. Lower explosion limit (% ,V/V): 2.6
23. Explosion upper limit (%,V/V): 12.5
24. Volume expansion coefficient (K-1 , 20ºC): 0.000695
25. Volume expansion coefficient (K-1, 55ºC): 0.000743
26. Vapor pressure (kPa, 55ºC): 0.19
27. Solubility: can be dissolved with water and ethanolMiscible with various organic solvents such as ether, chloroform, and acetone. Although its solubility in hydrocarbons, chlorinated hydrocarbons, and grease is small, its solubility is stronger than that of ethylene glycol.
28. Relative density (25℃, 4℃): 1.0328
29. Refractive index at room temperature (n25): 1.4314
30. Solubility parameter (J·cm-3)0.5: 29.516
31. van der Waals area (cm2 ·mol-1): 6.960×109
32. van der Waals volume (cm3·mol-1): 46.760
33. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -1902.55
34. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -421.29
35. Liquid phase standard combustion heat (enthalpy) (kJ ·mol-1): -1838.14
36. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -485.72
37. Liquid phase standard hot melt (J·mol-1·K-1): 189.9
Toxicological data
1. Toxicity classification Low toxicity2. Acute toxicity Oral – Rat LD50: 20000 mg/kg; Oral – Mouse LC50: 32000 mg/kg. 3. Irritation data Eyes – Rabbit 100 mg Mild 4. Low toxicity. It has minimal toxicity and irritation. The oral LD50 in rats is 32.5mL/kg. However, it is hemolytic and should not be used for intravenous injection. Like ethylene glycol, it carries the same risk of causing kidney disorders when added to food and drinks. Therefore some countries have banned its use in the food industry.
Ecological data
None
Molecular structure data
1. Molar refractive index: 18.97
2. Molar volume (cm3/mol): 73.4
3. Isotonic specific volume (90.2K ): 182.3
4. Surface tension (dyne/cm): 38.0
5. Polarizability (10-24cm3): 7.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 20.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Flammable liquids. It is hygroscopic and non-corrosive to metals. It reacts with dibasic acid to form polyester, reacts with nitric acid to form nitrate ester, and reacts with hydrochloric acid to form chlorohydrin. Heating with dilute sulfuric acid at 170°C converts it into propionaldehyde. Oxidation with nitric acid or chromic acid produces glycolic acid, oxalic acid, acetic acid, etc. Reacts with aldehydes to form acetal. Dehydration of 1,2-propanediol produces propylene oxide or polyethylene glycol.
2.Its toxicity and irritation are very small, and no victims have been found so far. Rats were given LD507000~8000mg/kg by intravenous injection and intraperitoneal injection, and LD502800mg/kg by mouth. However, there are also reports that when too high a dose is added to food and beverages, it may cause fatal drowsiness and kidney disorders.
3. Exist in tobacco leaves and smoke.
Storage method
1. This product should be sealed and stored in a cool and dry place. Keep away from fire and heat sources. It can be stored in containers made of iron, mild steel, copper, tin, stainless steel or resin-coated containers.
2. Although this product will not spontaneously ignite, it is combustible. It will not deteriorate after long-term storage, but it will absorb moisture when opened. Storage and transportation containers should be made of galvanized iron drums, aluminum or stainless steel. Store and transport according to general regulations on low-toxic chemicals.
Synthesis method
1. Propylene oxide direct hydration method is a pressurized non-catalytic hydrolysis method. It is produced by direct hydration of propylene oxide and water at 150-160°C and 0.78-0.98MPa pressure. The reaction product is evaporated and distilled to obtain the finished product.  2. Propylene oxide indirect hydration method is produced by indirect hydration of propylene oxide and water using sulfuric acid as a catalyst have to. 3. Direct catalytic oxidation of propylene.
2. Propylene oxide indirect hydration method is produced by indirect hydration of propylene oxide and water using sulfuric acid as a catalyst have to. 3. Direct catalytic oxidation of propylene.  4. Using 1,2-dichloropropane as raw material This method has two process routes: 1. The first is that dichloropropane is directly hydrolyzed into propylene glycol in a weak alkali aqueous solution; the second is that dichloropropane reacts with carboxylate to first form an ester, and the ester is then hydrolyzed into propylene glycol. (1) Direct hydrolysis process: Add 1,2-dichloropropane, water, sodium bicarbonate and cetyltributylphosphonium bromide into the reaction kettle, and react at 100°C under a carbon dioxide partial pressure of 1.0MPa 18h, 80% propylene glycol is obtained. Control the feeding speed of dichloropropane, that is, the feeding speed is fast at high temperature and slow at low temperature. Example: Add 60g calcium carbonate and 150g water into a 300ml autoclave, stir and heat to 230°C, continuously add dichloropropane at a rate of 0.03g/(min·100gH2O) for 11.5h; continue stirring at this temperature for 30min, and then quench At room temperature, the propylene glycol yield is about 95%. By controlling the temperature within 130-300°C and changing the feed rate of dichloropropane accordingly, the yield of propylene glycol can reach over 95%. (2) Two-step hydrolysis process: The raw materials are first reacted in a kettle reactor. After the dichloropropane reaches a certain conversion rate, the material is then pumped into a plug flow reactor to continue the reaction, and finally hydrolyzed into propylene glycol. Example: Add 606kg of dichloropropane into a 2 cubic meter reaction kettle, then add 800kg of sodium acetate, 556kg of 1,2-propanediol, 10kg of acetic acid and 1kg of water, stir and raise the temperature to 180°C, cool to 120°C after 4 hours, and extract the material. After the preheater is heated to 180°C, it passes through a plug flow reactor with a length of 400m, an inner diameter of 25mm, and a volume of 230L at a speed of 500L/h. The product is collected in the second stirred tank and cooled to room temperature. The analyzed product is: 44kg dichloropropane, 334kg propylene glycol, 32kg sodium acetate, 44kg acetic acid, 234kg 1,2-diacetoxypropane, 693kg propylene glycol monoacetate, 45kg 1-chloropropene, 547kg NACL and 1kg water.
4. Using 1,2-dichloropropane as raw material This method has two process routes: 1. The first is that dichloropropane is directly hydrolyzed into propylene glycol in a weak alkali aqueous solution; the second is that dichloropropane reacts with carboxylate to first form an ester, and the ester is then hydrolyzed into propylene glycol. (1) Direct hydrolysis process: Add 1,2-dichloropropane, water, sodium bicarbonate and cetyltributylphosphonium bromide into the reaction kettle, and react at 100°C under a carbon dioxide partial pressure of 1.0MPa 18h, 80% propylene glycol is obtained. Control the feeding speed of dichloropropane, that is, the feeding speed is fast at high temperature and slow at low temperature. Example: Add 60g calcium carbonate and 150g water into a 300ml autoclave, stir and heat to 230°C, continuously add dichloropropane at a rate of 0.03g/(min·100gH2O) for 11.5h; continue stirring at this temperature for 30min, and then quench At room temperature, the propylene glycol yield is about 95%. By controlling the temperature within 130-300°C and changing the feed rate of dichloropropane accordingly, the yield of propylene glycol can reach over 95%. (2) Two-step hydrolysis process: The raw materials are first reacted in a kettle reactor. After the dichloropropane reaches a certain conversion rate, the material is then pumped into a plug flow reactor to continue the reaction, and finally hydrolyzed into propylene glycol. Example: Add 606kg of dichloropropane into a 2 cubic meter reaction kettle, then add 800kg of sodium acetate, 556kg of 1,2-propanediol, 10kg of acetic acid and 1kg of water, stir and raise the temperature to 180°C, cool to 120°C after 4 hours, and extract the material. After the preheater is heated to 180°C, it passes through a plug flow reactor with a length of 400m, an inner diameter of 25mm, and a volume of 230L at a speed of 500L/h. The product is collected in the second stirred tank and cooled to room temperature. The analyzed product is: 44kg dichloropropane, 334kg propylene glycol, 32kg sodium acetate, 44kg acetic acid, 234kg 1,2-diacetoxypropane, 693kg propylene glycol monoacetate, 45kg 1-chloropropene, 547kg NACL and 1kg water.
Purpose
1. Propylene glycol is an important raw material for unsaturated polyester, epoxy resin, polyurethane resin, plasticizer, and surfactant. Its usage accounts for about 45% of the total consumption of propylene glycol. This unsaturated polyol Esters are used extensively in surface coatings and reinforced plastics. Propylene glycol is widely used as a hygroscopic agent, antifreeze, lubricant and solvent in the food, pharmaceutical and cosmetic industries due to its good viscosity, hygroscopicity and non-toxic properties. In the food industry, propylene glycol reacts with fatty acids to form propylene glycol fatty acid esters, which are mainly used as food emulsifiers; propylene glycol is an excellent solvent for condiments and pigments. Due to its low toxicity, it is used as a solvent for spices and food colorings in the food industry. Propylene glycol is commonly used in the pharmaceutical industry as a solvent, softener and excipient in the manufacture of various ointments and ointments. In the pharmaceutical industry, it is used as a solvent for blending agents, preservatives, ointments, vitamins, penicillins, etc. Because propylene glycol has good miscibility with various fragrances, it is also used as a solvent and softener in cosmetics. Propylene glycol is also used as a tobacco humidifier, antifungal agent, food processing equipment lubricant, and solvent for food marking ink. Aqueous solutions of propylene glycol are effective antifreeze agents. It is also used as tobacco wetting agent, antifungal agent, fruit ripening preservative, antifreeze and heat carrier.
2.Used in organic synthesis as solvent, dehydrating agent, plasticizer, antifreeze, and gas chromatography fixative.
3.Commonly used organic synthetic raw materials for the manufacture of unsaturated polyester resin. It can also be used as emulsifier, preservative and antifreeze. It is also used in the manufacture of alkyd resins, polypropylene glycol, plasticizers, surfactants and lubricants. Due to its good hygroscopicity and low toxicity, it is used in the pharmaceutical industry as a solvent for blenders, preservatives, ointments, ointments, pills and vitamins, as well as softeners and excipients. Used as a solvent for spices, condiments and food colorings in the food industry. It is also used as tobacco humidifier, antifungal agent, fruit ripening preservative, coating film-forming additive, antifreeze and heat transfer medium. It is also often used as a substitute for ethanol and glycerin, and can be used as a wetting agent in combination with glycerin or sorbitol in toothpaste and cosmetics.
extended-reading:https://www.bdmaee.net/cas-1696-20-4/extended-reading:https://www.cyclohexylamine.net/addocat-106-teda-l33b-dabco-polycat/extended-reading:https://www.newtopchem.com/archives/779extended-reading:https://www.bdmaee.net/polyurethane-rigid-foam/extended-reading:https://www.newtopchem.com/archives/573extended-reading:https://www.newtopchem.com/archives/39978extended-reading:https://www.newtopchem.com/archives/206extended-reading:https://www.bdmaee.net/cas-108-01-0-2/extended-reading:https://www.newtopchem.com/archives/40383extended-reading:https://www.cyclohexylamine.net/dabco-ne500-non-emission-amine-catalyst-ne500/
















 4. Using 1,2-dichloropropane as raw material This method has two process routes: 1. The first is that dichloropropane is directly hydrolyzed into propylene glycol in a weak alkali aqueous solution; the second is that dichloropropane reacts with carboxylate to first form an ester, and the ester is then hydrolyzed into propylene glycol. (1) Direct hydrolysis process: Add 1,2-dichloropropane, water, sodium bicarbonate and cetyltributylphosphonium bromide into the reaction kettle, and react at 100°C under a carbon dioxide partial pressure of 1.0MPa 18h, 80% propylene glycol is obtained. Control the feeding speed of dichloropropane, that is, the feeding speed is fast at high temperature and slow at low temperature. Example: Add 60g calcium carbonate and 150g water into a 300ml autoclave, stir and heat to 230°C, continuously add dichloropropane at a rate of 0.03g/(min·100gH2O) for 11.5h; continue stirring at this temperature for 30min, and then quench At room temperature, the propylene glycol yield is about 95%. By controlling the temperature within 130-300°C and changing the feed rate of dichloropropane accordingly, the yield of propylene glycol can reach over 95%. (2) Two-step hydrolysis process: The raw materials are first reacted in a kettle reactor. After the dichloropropane reaches a certain conversion rate, the material is then pumped into a plug flow reactor to continue the reaction, and finally hydrolyzed into propylene glycol. Example: Add 606kg of dichloropropane into a 2 cubic meter reaction kettle, then add 800kg of sodium acetate, 556kg of 1,2-propanediol, 10kg of acetic acid and 1kg of water, stir and raise the temperature to 180°C, cool to 120°C after 4 hours, and extract the material. After the preheater is heated to 180°C, it passes through a plug flow reactor with a length of 400m, an inner diameter of 25mm, and a volume of 230L at a speed of 500L/h. The product is collected in the second stirred tank and cooled to room temperature. The analyzed product is: 44kg dichloropropane, 334kg propylene glycol, 32kg sodium acetate, 44kg acetic acid, 234kg 1,2-diacetoxypropane, 693kg propylene glycol monoacetate, 45kg 1-chloropropene, 547kg NACL and 1kg water.
4. Using 1,2-dichloropropane as raw material This method has two process routes: 1. The first is that dichloropropane is directly hydrolyzed into propylene glycol in a weak alkali aqueous solution; the second is that dichloropropane reacts with carboxylate to first form an ester, and the ester is then hydrolyzed into propylene glycol. (1) Direct hydrolysis process: Add 1,2-dichloropropane, water, sodium bicarbonate and cetyltributylphosphonium bromide into the reaction kettle, and react at 100°C under a carbon dioxide partial pressure of 1.0MPa 18h, 80% propylene glycol is obtained. Control the feeding speed of dichloropropane, that is, the feeding speed is fast at high temperature and slow at low temperature. Example: Add 60g calcium carbonate and 150g water into a 300ml autoclave, stir and heat to 230°C, continuously add dichloropropane at a rate of 0.03g/(min·100gH2O) for 11.5h; continue stirring at this temperature for 30min, and then quench At room temperature, the propylene glycol yield is about 95%. By controlling the temperature within 130-300°C and changing the feed rate of dichloropropane accordingly, the yield of propylene glycol can reach over 95%. (2) Two-step hydrolysis process: The raw materials are first reacted in a kettle reactor. After the dichloropropane reaches a certain conversion rate, the material is then pumped into a plug flow reactor to continue the reaction, and finally hydrolyzed into propylene glycol. Example: Add 606kg of dichloropropane into a 2 cubic meter reaction kettle, then add 800kg of sodium acetate, 556kg of 1,2-propanediol, 10kg of acetic acid and 1kg of water, stir and raise the temperature to 180°C, cool to 120°C after 4 hours, and extract the material. After the preheater is heated to 180°C, it passes through a plug flow reactor with a length of 400m, an inner diameter of 25mm, and a volume of 230L at a speed of 500L/h. The product is collected in the second stirred tank and cooled to room temperature. The analyzed product is: 44kg dichloropropane, 334kg propylene glycol, 32kg sodium acetate, 44kg acetic acid, 234kg 1,2-diacetoxypropane, 693kg propylene glycol monoacetate, 45kg 1-chloropropene, 547kg NACL and 1kg water.