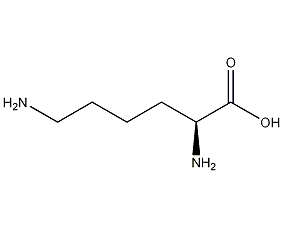
Structural formula
| Business number | 02CD |
|---|---|
| Molecular formula | C4H6N4O3 |
| Molecular weight | 158.12 |
| label |
2,5-Dichloro-4-imidazolidinylurea, 1-ureidodiazapentane-2,4-dione, Glyoxylic (acid) diureide, (2,5-Dioxo-4-imidazolidinyl)urea, Glyoxylic diureide, Psoralon, Septalan, 5-Ureidohydantoin, Heterocyclic compounds |
Numbering system
CAS number:97-59-6
MDL number:MFCD00005260
EINECS number:202-592-8
RTECS number:YT1600000
BRN number:102364
PubChem ID:None
Physical property data
1. Appearance: Monoclinic flake or prismatic crystal
2. Melting point (decomposition, ℃): 238
3. Solubility: can be dissolved in hot water, Hot ethanol and dilute sodium hydroxide solution, slightly soluble in water and ethanol (1g dissolved in 190ml water, 500ml ethanol), almost insoluble in butyl ether and chloroform.
4. Saturated aqueous solution pH: 5.5
Toxicological data
None yet
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 33.42
2. Molar volume (cm3/mol): 95.7
3. Isotonic specific volume (90.2K ): 288.5
4. Surface tension (dyne/cm): 82.6
5. Polarizability (10-24cm3): 13.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -2.2
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 3
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 24
6. Topological molecular polar surface area 113
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 225
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Odorless and tasteless. It is stable in dry air, but will be destroyed when boiled in water for a long time or in strong alkali.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. 2. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Allantoin exists in cystic fluid, fetal urine and some…in things. But extracting vesicles from these substances is too expensive. The current methods for synthesizing allantoin include: potassium permanganate oxidation of urea acid method: heating dichloroacetic acid and urea to synthesize allantoin; direct condensation method of glyoxylic acid and urea. 1. The process using dichloroacetic acid and urea as raw materials is as follows: Put sodium methoxide solution and methanol into the reaction tank, heat to 40-50°C, slowly add dichloroacetic acid dropwise, and then reflux for 2 hours after the addition. Cool to room temperature, filter, wash with methanol, combine the filtrate and add sodium dimethoxylate methanol solution. The solution was reduced to dryness under reduced pressure, added 2.8 parts of hydrochloric acid, heated on a water bath and stirred into a paste. Then raise the temperature to 90°C, then cool to about 10°C, filter, add 0.25 parts of hydrochloric acid and urea to the small liquid, heat to dissolve, and react at 80°C for 2 hours. Cool and crystallize, then maintain it at 0°C for more than 3 hours, centrifuge, wash with water, spin dry, and dry to obtain a fine product. The crude product is recrystallized with 15 times of water to obtain allantoin. The total yield of dichloroacetic acid is 30.3%. 2. Direct condensation of glyoxylic acid and urea (glyoxylic acid is obtained by oxidation of glyoxal) Operation example: (1) Oxidation in a 500ml four-neck bottle equipped with mechanical stirring, condenser, thermometer and dropping funnel, Add 193g (1mol) 30% glyoxal aqueous solution, and add 135.5g (1mol) 45% nitric acid dropwise with stirring while controlling the internal temperature to 40±2°C. After the addition, continue the stirring reaction at this temperature for 2-3 hours until the red-brown oxidation ceases. Escape, the reaction solution turns blue-green and disappears. Change to a simple distillation device, distill out about 125g of water at about 2.76kPa and the external temperature does not exceed 60°C. The concentrated solution is allowed to stand at room temperature overnight. The precipitated oxalic acid crystals (19g after drying, totaling 0.21mol) have a concentration of 39%. ). Continue to stir and react at an internal temperature of 40±2°C for about 8 hours, and the oxidation reaction will be completed. (2) Condensation In a 500ml three-necked flask equipped with a mechanical stirrer, condenser and thermometer, add the above 150g glyoxylic acid aqueous solution, then add 170g (2.83mol) urea and 23.3g concentrated hydrochloric acid, and heat with stirring until the internal temperature reaches 80 ℃. After about 15 minutes, the urea dissolves and the reactant becomes a transparent liquid. After about 30-45 minutes, the reactant becomes white and turbid, and then the white precipitate gradually increases. Stir for 1 hour at an internal temperature of 80°C. Stop heating and cool the reactant to room temperature. Use a Buchner funnel to collect the white precipitate and precipitate with cold water several times to obtain crude allantoin. Recrystallize with 1000ml distilled water. 60-63% of white fine crystalline allantoin is obtained, with a melting point of 236°C (decomposition). Based on glyoxal, the allantoin yield is 38-40% of the theory (weight yield is 103-108%).
2. It is synthesized by heating using dichloroacetic acid and urea as raw materials. Add sodium methoxide solution and methanol to the reaction kettle, heat to 40-50°C, slowly add dichloroacetic acid dropwise, and reflux for 2 hours after the addition. Cool to room temperature, filter, wash with formic acid, and combine the filtrate to obtain a methanol solution of sodium dimethoxyacetate. Concentrate the solution to dryness under reduced pressure, add 2.8 parts of hydrochloric acid, heat over water and stir to form a paste. Then raise the temperature to 90°C, then cool to about 10°C, filter, add 0.25 parts of hydrochloric acid and urea to the filtrate, heat to dissolve, and react at 80°C for 2 hours. Cool and crystallize, then maintain at 0°C for more than 3 hours, centrifuge, wash with water, spin and dry to obtain crude product. The crude product is recrystallized with 15 times of water to obtain allantoin. The response is as follows:

Purpose
1. Used for skin care, oral products, anti-allergy, treating skin ulcers and promoting wound healing.
2.Allantoin has the effect of stimulating the growth of healthy tissue and healing injuries. It is often used in the manufacture of medical ointments to treat wounds and ulcers. This product also promotes the water absorption capacity of the outermost tissues of the skin and hair, protects against light, and has antioxidant effects, making the skin soft, elastic, shiny, and preventing dryness and cracking. Since allantoin has the effect of softening keratin, it can keep hair from splitting or breaking. It can be used as an additive in skin care and hair conditioner cosmetics. It can also be used as a plant growth promoter in agriculture. Allantoin is also a biochemical reagent.
3. Digestive system drugs are used to treat gastric ulcers, duodenal ulcers, chronic gastritis, antral gastritis and gastritis caused by drugs. They are also used externally to eliminate ulcers. It is also widely used as a quality improver in daily chemicals such as beauty cosmetics, shampoo, soap, toothpaste, astringent liquid, and acne liquid.
extended-reading:https://www.bdmaee.net/monobutyltin-oxide-2/extended-reading:https://www.bdmaee.net/nn-dicyclohexylmethylamine-3/extended-reading:https://www.newtopchem.com/archives/39811extended-reading:https://www.bdmaee.net/cas-23850-94-4/extended-reading:https://www.cyclohexylamine.net/trimethylhydroxyethyl-bisaminoethyl-ether-jeffcat-zf-10/extended-reading:https://www.bdmaee.net/toyocat-dt-strong-foaming-catalyst-pentamethyldiethylenetriamine-tosoh/extended-reading:https://www.morpholine.org/delayed-catalyst-for-foaming-dabco-dc2-polyurethane-catalyst-dabco-dc2/extended-reading:https://www.newtopchem.com/archives/40000extended-reading:https://www.newtopchem.com/archives/884extended-reading:https://www.newtopchem.com/archives/43960