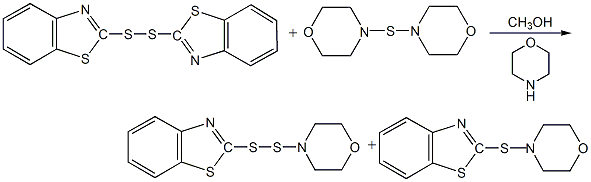
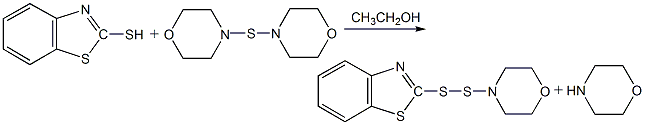
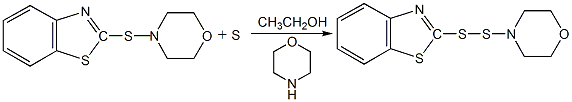
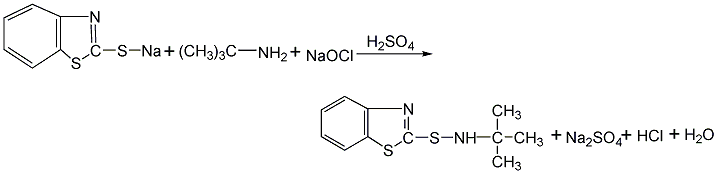
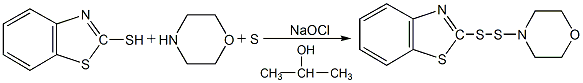Structural formula
| Physical competition number | 0254 |
|---|---|
| Molecular formula | C19H16ClNO4 |
| Molecular weight | 357.79 |
| label |
C.I. Ice dye coupling component 12, Naphthol AS-ITR, Naphto AS-ITR, Acco Naf-Sol AS-ITR, Amanil Naphthol AS-ITR, Fluorescent dyes |
Numbering system
CAS number:92-72-8
MDL number:MFCD00021635
EINECS number:202-182-9
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Properties: light gray or green powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 193-194
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 94.7kpa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in acetone, soluble in pyridine , ethanol and benzene, insoluble in sodium carbonate solution. It is a yellow solution with green fluorescence in sulfuric acid; it is a yellow solution in sodium hydroxide. Stable to air.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 98.80
2. Molar volume (cm3/mol): 260.8
3. Isotonic specific volume (90.2K ): 714.9
4. Surface tension (dyne/cm): 56.4
5. Polarizability (10-24cm3): 39.16
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 4.8
2. Hydrogen bond donor�Number of bonds: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Tautomers Number: 9
6. Topological molecule polar surface area 67.8
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 463
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain Number of atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15 .Number of covalent bond units: 1
Properties and stability
Stable to air, dip dyeing temperature is 30ºC and pad dyeing temperature is 90ºC.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
Using 2,3-acid and 2,4-dimethoxy-5-chloroaniline as raw materials, 2,3-acid is first made into sodium salt in chlorobenzene medium, and then mixed with 2,4-acid in the presence of PCl3 Dimethoxy-5-chloroaniline is condensed, and the finished product is obtained through neutralization, distillation, filtration and drying.
Purpose
Nathophenol AS-ITR is mainly used as a dyeing primer for cotton to dye red. For example, it can be used with red base ITR and red base KL to dye bright pink. High affinity to cotton. It is also used for dyeing viscose fiber, silk, nylon, printing cotton and manufacturing lake pigments.
extended-reading:https://www.newtopchem.com/archives/1761extended-reading:https://www.newtopchem.com/archives/category/products/page/89extended-reading:https://www.bdmaee.net/composite-amine-catalyst/extended-reading:https://www.bdmaee.net/cas-3855-32-1/extended-reading:https://www.bdmaee.net/dabco-mp602-catalyst-cas31506-44-2-evonik-germany/extended-reading:https://www.bdmaee.net/catalyst-smp/extended-reading:https://www.bdmaee.net/nt-cat-dbu-catalyst-cas6674-22-2-newtopchem/extended-reading:https://www.newtopchem.com/archives/44922extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/27.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/83