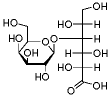
Structural formula
| Business number | 017F |
|---|---|
| Molecular formula | [N(C2H5)4]Cl |
| Molecular weight | 165.7 |
| label |
Tetraethylammonium chloride, TEA chloride, Reagents for genetic engineering research, catalyst |
Numbering system
CAS number:56-34-8
MDL number:MFCD00011828
EINECS number:200-267-5
RTECS number:24277874
BRN number:3563247
PubChem number:24277874
Physical property data
1. Properties: Hygroscopic crystals, easy to form tetrahydrate.
2. Density (g/mL, 25/4℃): 1.08
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 37.5
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, ethanol, chloroform and acetone, Slightly soluble in benzene. The pH of a 10% aqueous solution is 6.48, and the pH remains unchanged when heated to 95°C for 28 hours.
Toxicological data
1. Acute toxicity: human intravenous TDLo: 5mg/kg; rat oral LD50: 2630mg/kg; rat subcutaneous LD50: 200mg/kg; rat intravenous LD50: 56mg/kg; rat intramuscular LDLo: 110mg/kg ; Mouse oral LD50: 833mg/kg; Mouse abdominal LD50: 65mg/kg; Mouse subcutaneous LDLo: 120mg/kg; Mouse intravenous LD50: 37mg/kg; Dog intravenous LD50: 36mg/kg; Dog intramuscular LD50 : 58mg/kg; frog subcutaneous LDLo: 3488mg/kg2, other multiple dose toxicity: dog intramuscular TDLo: 1275mg/kg/22D-I
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 47.5
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain atoms Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 2
Properties and stability
Easily soluble in water, ethanol, chloroform and acetone, slightly soluble in benzene.
Storage method
This product should be sealed and stored in a dry and dark place.
Synthesis method
Neutralize 10% tetraethylammonium hydroxide aqueous solution with hydrochloric acid to obtain a tetraethylammonium chloride solution. The aqueous solution is evaporated to dryness, and the residue is recrystallized in an acetonitrile-acetone mixed solvent to obtain tetraethylammonium chloride. The product is vacuum dried in the presence of phosphorus pentoxide.
Purpose
Phase transfer catalysts for polymerization reactions. Membranes carry electrolytes for research. Test for antimony and bismuth, and determine gold. Polarographic analysis.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-8.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/9.jpgextended-reading:https://www.bdmaee.net/pentamethyldiethylenetriamine-cas-3030-47-5-pc5/extended-reading:https://www.morpholine.org/category/morpholine/page/5392/extended-reading:https://www.bdmaee.net/pentamethyldipropylenetriamine-cas3855-32-1-nnnnn-pentamethyldipropylenetriamine/extended-reading:https://www.bdmaee.net/22-dimorpholinodiethylether-3/extended-reading:https://www.newtopchem.com/archives/39593extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/07/12.jpgextended-reading:https://www.morpholine.org/category/morpholine/page/5389/extended-reading:https://www.newtopchem.com/archives/40401