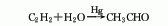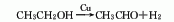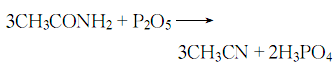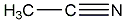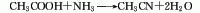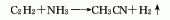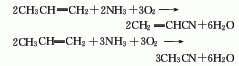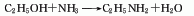Structural formula
| Business number | 01HZ |
|---|---|
| Molecular formula | CH2Cl2 |
| Molecular weight | 85 |
| label |
dichloromethylene, methylene chloride, Methylene dichloride, Methylene dichloride, DCM |
Numbering system
CAS number:75-09-2
MDL number:MFCD00000881
EINECS number:200-838-9
RTECS number:PA8050000
BRN number:1730800
PubChem number:24856423
Physical property data
1. Properties: colorless and transparent liquid with aromatic odor. [1]
2. Melting point (℃): -95[2]
3. Boiling point (℃): 39.8[3]
4. Relative density (water = 1): 1.33[4]
5. Relative vapor Density (air=1): 2.93[5]
6. Saturated vapor pressure (kPa): 46.5 (20℃)[6]
7. Heat of combustion (kJ/mol): -604.9[7]
8. Critical temperature (℃): 237[8]
9. Critical pressure (MPa): 6.08[9]
10. Octanol/water partition coefficient: 1.25 [10]
11. Flash point (℃): -4[11]
12. Ignition temperature (℃): 556[12]
13. Explosion upper limit (%): 22[13]
14. Explosion lower limit (% ): 14[14]
15. Solubility: Slightly soluble in water, soluble in ethanol and ether. [15]
16. Viscosity (mPa·s, 20ºC): 0.425
17. Relative density (25℃, 4℃): 1.3162
18. Ignition point (ºC): 662
19. Heat of evaporation (KJ/mol, b.p.): 329.5
20. Heat of fusion (KJ/mol) : 4.187
21. Heat of formation (KJ/mol, 25ºC, liquid): 121.54
22. Heat of combustion (KJ/kg, 25ºC, liquid): 558.27
23. Specific heat capacity (KJ/(kg·K), 20ºC): 0.992
24. Electrical conductivity (S/m, 25ºC): 4.3×10-11
25. Vapor pressure (kPa, 0ºC): 19.7
26. Vapor pressure (kPa, 10ºC): 30.6
27. Vapor pressure (kPa, 20ºC): 46.5
28. Vapor pressure (kPa, 30ºC): 68.2
29. Vapor pressure (kPa, 35ºC): 80.00
30. Body Expansion coefficient (K-1, 10~40ºC, liquid): 0.00137
31. Refractive index at room temperature (n25): 1.4213
32. Eccentricity factor: 0.192
33. Lennard-Jones parameter (A): 9.951
34. Lennard-Jones parameter (K): 150.5
35. Solubility parameter (J·cm-3)0.5: 20.378
36.van der Waals area (cm 2·mol-1): 4.990×109
37. van der Waals volume (cm3 sup>·mol-1): 34.710
38. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -95.4
39. Gas phase standard entropy (J·mol-1·K-1): 270.44
40. Gas phase standard generation Free energy (kJ·mol-1): -68.8
41. Gas phase standard hot melt (J·mol-1·K -1): 50.88
42. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -124.26
43. Liquid phase standard entropy (J·mol-1·K-1): 178.7
44. Liquid phase standard free energy of formation (kJ·mol-1): -70.42
45. Liquid phase standard hot melt (J·mol-1·K-1): 100.0
Toxicological data
1. Acute toxicity[16]
LD50: 1600~2000mg/kg (rat oral )
LC50: 88000mg/m3 (rat inhalation, 1/2h)
2. Irritation [17]
Rabbit transdermal: 810mg (24h), severe stimulation.
Rabbit eye: 162mg, moderate irritation.
3. Subacute and chronic toxicity[18]
Rat inhalation 4.69g /m3, 8 hours a day, 75 days in total, no pathological changes. As exposure time increased, there was mild liver atrophy, steatosis, and cellular infiltration.
4. Mutagenicity[19] Microbial mutagenicity: Salmonella typhimurium 5700ppm. DNA inhibition: human fibroblasts 5000ppm (1h) (continuous). DNA damage: Hamster ovary 3000ppm. Sister chromatid exchange: hamster lung 5000ppm (1h) (continuous)
5. Teratogenicity[20] Rats were given the lowest toxic dose (TCLo) of 1250ppm (7h) by inhalation 6 to 15 days after pregnancy, causing developmental malformations in the musculoskeletal system and genitourinary system.
6. Carcinogenicity[21] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
7. Others[22] The lowest inhalation toxic concentration for rats (TCLo): 1250ppm (7h) (pregnant 6~15 days), causing abnormal musculoskeletal development and abnormal development of the genitourinary system.
Ecological data
1. Ecotoxicity[23]
LC50: 193mg/L (96h) (fathead minnow , dynamic); 310mg/L (96h) (fathead minnow, static); 200~250mg/L (96h) (bluegill, static); 224mg/L (48h) (water fleas); 256mg/L ( 96h) (sugar shrimp)
2. Biodegradability[24]
Good Aerobic biodegradation (h): 168~672
Anaerobic biodegradation (h): 672~2688
3. Non-biodegradability [25]
Photolysis maximum light absorption wavelength range (nm): 220~250
Photooxidation half-life in air – high (h): 458~4584
First-grade hydrolysis half-life (h): 704a
4. Other harmful effects[26] This substance is harmful to the environment and has an accumulation effect in groundwater. Special attention should be paid to aquatic life. Attention should also be paid to atmospheric pollution.
Molecular structure data
1. Molar refractive index: 16.38
2. Molar volume (cm3/mol): 67.8
3. Isotonic specific volume (90.2K ): 148.8
4. Surface tension (dyne/cm): 23.1
5. Polarizability: 6.49
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Topological molecular polar surface area (TPSA): 0
6. Number of heavy atoms: 3
7. Surface charge: 0
8. Complexity: 2.8
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 0
11. The number of uncertain atomic stereocenters: 0
12. The number of determined chemical bond stereocenters: 0
13. Uncertain chemical bond stereocenters Number of structural centers: 0
14. Number of covalent bond units: 1
Properties and stability
1. It has very little toxicity and recovers quickly after poisoning, so it can be used as an anesthetic. Irritating to skin and mucous membranes. Oral LD50 in young adult rats: 1.6mL/kg. The maximum allowable concentration in the air is 500×10-6. Gas masks should be worn during operation. When poisoning is discovered, leave the scene immediately and receive symptomatic treatment. The smallest among the chlorides of methane. The vapor is highly anesthetic, and inhaling large amounts can cause acute poisoning, with symptoms such as nasal pain, headache, and vomiting. Chronic poisoning can cause dizziness, fatigue, loss of appetite, impaired hematopoietic function, and reduced red blood cells. Liquid methylene chloride can cause dermatitis when it comes into contact with the skin. Rats died after inhaling vapor with a concentration of 90.5g/m3 for 90 minutes. The olfactory threshold concentration is 522mg/m3, and the maximum allowable concentration in the workplace is 1740mg/m3.
2. Stability[27] Stable
3. Incompatible substances[28] Alkali metals, aluminum
4. Conditions to avoid contact [29] Light, humid air
5. Polymerization hazard[30] No polymerization
6. Decomposition products[31] Hydrogen chloride, phosgene
Storage method
Storage Precautions[32] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. Keep container tightly sealed. Should be stored separately from alkali metals and food chemicals, and avoidMixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Methane chlorination method: After mixing methane, chlorine and circulating gas, they are sent to the reactor for thermal chlorination reaction at 380~400°C. After the gas reaction product is cooled to 70-80°C by the cooler, it is sent to the hydrogen chloride absorption tower, where the hydrogen chloride in the gas product is absorbed and removed with water, and then sent to the alkali washing tower, where it is neutralized with alkali liquid to remove residual hydrochloric acid and free chlorine. The purified gas product is compressed, cooled, and condensed into crude chloride. The crude chloride is sent to the distillation process, and methyl chloride, methylene chloride and chloroform are steamed out through each distillation tower. Except for a part of the non-condensable gas that is vented, the rest is mixed with new materials for recycling. The controlled operating conditions are reaction temperature 400-420°C, ingredient ratio CH4:Cl2=4:1, and products based on methylene chloride can be obtained.

2. Methanol ammoniation method: After the methanol is gasified in the gasifier, it is mixed with the hydrogen chloride separated in the subsequent reaction, and then passed through the catalyst alumina or suboxone chloride or zinc chloride with activated carbon or pumice as the carrier. Phosphoric acid supported on the activated carbon can also be used as the catalyst. , react in a hydrochlorinator at 350°C to generate methyl chloride. The gaseous reaction product is washed with cold water and sodium hydroxide solution in a water washing tower or an alkali washing tower respectively. After removing unreacted methanol and hydrogen chloride, it is sent to the dehydrogenation tower and washed again with sulfuric acid to remove the dimethyl ether and dimethyl ether generated during the reaction. water to obtain methyl chloride. Methyl chloride reacts with chlorine at 410 to 420°C to produce dichloromethane and chloroform, as well as a small amount of carbon tetrachloride. After quenching, it is divided into a liquid phase containing dichloroethane and a gas phase containing uncondensed hydrogen chloride, unreacted methyl chloride, and chlorine. The gas phase is separated to separate hydrogen chloride, methyl chloride and chlorine for recycling. The liquid phase is first sent to the absorption tower and washed with dilute alkali and water to remove carbon tetrachloride, and then sent to the rectification tower to obtain dichloromethane and chloroform respectively through azeotropic distillation and rectification.

3. Methyl chloride chloride Methane chlorination has two processes: photochlorination and thermal chlorination.
Photochlorination is the reaction of methyl chloride and chlorine under 4000kW light. The reaction product is washed with alkali, compressed, condensed, dried and distilled to obtain the finished product.
Thermal chlorination is to mix methyl chloride and chlorine gas according to (2~2.5):1 (mass), and react at a reaction temperature of 400°C and a reaction pressure of 0.2MPa. The reaction product is washed with water, alkali washed, compressed, condensed, dried and distilled to obtain the finished product. ![]()
4. Use industrial methylene chloride as raw material , wash several times with concentrated sulfuric acid 1/8 to 1/10 of the raw material volume until the acid layer is colorless, then wash once with 5% sodium hydroxide solution, the dosage is 1/5 of the raw material volume, and wash twice with water. Then it is dried with anhydrous calcium chloride, the clear liquid is sucked out and then distilled, and the middle fraction is collected to obtain pure methylene chloride.
Purpose
1. In addition to being used in organic synthesis, this product is also widely used as a solvent in cellulose acetate film forming, cellulose triacetate spinning, petroleum dewaxing, aerosols and the production of antibiotics, vitamins and steroids. As well as cleaning, degreasing and release agents for metal surface paint layers.
2. Used for grain fumigation and refrigeration of low-pressure freezers and air-conditioning devices. It is used as an auxiliary blowing agent in the production of polyether urethane foam and as a blowing agent for extruded polysulfone foam.
3. Used as solvent, extraction agent and mutagen. For plant genetic research.
4. It has good dissolving power. It is a low-toxic, non-flammable and low-boiling point solvent among commonly used industrial solvents. It has good dissolving power for many resins, paraffin and fats. Mainly used as paint stripper, petroleum dewaxing solvent, extractant for thermally unstable substances, extractant for lanolin from wool and edible oil from coconut, and solvent for cellulose triacetate film film. It is also widely used in the manufacturing and processing of acetate fiber and vinyl chloride fiber, as well as the manufacturing of fire extinguishing agents, refrigerants, methenamine, etc.
5. Used in the electronics industry. Commonly used as a cleaning and degreasing agent.
6. Because it has an extremely low boiling point and is a flame-retardant solvent, it is widely used. In addition to being used as a cleaning solvent for aircraft engines, precision machinery, etc., it can also be used as a stripper for coatings. It can also be properly mixed with other solvents and used in various industrial cleanings.
7. It is also used as an ethyl fiber solvent, dental local anesthetic, refrigerant and fire extinguishing agent. It is a commonly used eluent for chromatographic separation and a common solvent for extraction separation.
8. Used as a solvent in the resin and plastic industry. [33]
extended-reading:https://www.newtopchem.com/archives/39733extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/123-1.jpgextended-reading:https://www.newtopchem.com/archives/40251extended-reading:https://www.newtopchem.com/archives/43932extended-reading:https://www.newtopchem.com/archives/582extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/77.jpgextended-reading:https://www.newtopchem.com/archives/575extended-reading:https://www.cyclohexylamine.net/pentamethyldiethylenetriamine-cas-3030-47-5/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/72.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/15.jpg