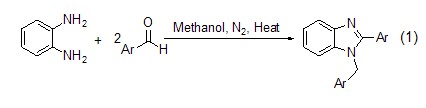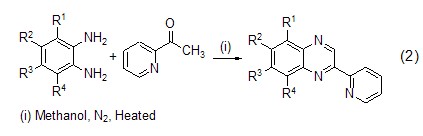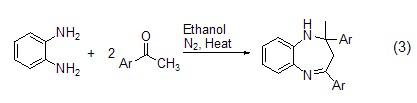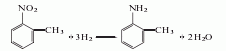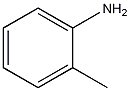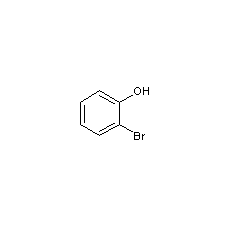
Structural formula
| Business number | 029D |
|---|---|
| Molecular formula | C6H5BrO |
| Molecular weight | 173.01 |
| label |
2-bromophenol, o-bromophenol, o-bromophenol, ο-Bromophenol, BrC6H4OH, Multifunctional solvents, drug intermediates, aromatic compounds |
Numbering system
CAS number:95-56-7
MDL number:MFCD00002146
EINECS number:202-432-7
RTECS number:SJ7875000
BRN number:1905115
PubChem number:24847983
Physical property data
1. Properties: yellow to red oily liquid with unpleasant odor. [1]
2. Melting point (℃): 5.6[2]
3. Boiling point (℃): 195 [3]
4. Relative density (water = 1): 1.49[4]
5. Saturated vapor pressure (kPa): 1.73 (87.3℃)[5]
6. Octanol/water partition coefficient: 2.35[6]
7. Flash point (℃): 42.22[7]
8. Solubility: slightly soluble in water, soluble in ethanol, ether, etc. [8]
Toxicological data
1. Acute toxicity: Mouse oral LD50: 652mg/kg; Mouse abdominal LD50: 633mg/kg; Guinea pig subcutaneous LDLo: 1500mg/kg; Mammalian oral LD50: 650mg/kg; Mammalian oral LD50: 650mg/kg; Inhalation LC50: 1mg/m3;
2. Irritating to skin and mucous membranes.
3. Acute toxicity [9] LD50: 652mg/kg (mouse oral)
Ecological data
1. Ecotoxicity[10]
LC50: 16mg/L (48h) (medaka)
IC50: 78mg/L (72h) (algae)
2. Biodegradability No data available
3. Non-biodegradability No data yet
4. Bioaccumulation [11] BCF: 20~33 (carp, contact concentration 30ppb, contact time 6 weeks); 23~41 (carp, exposure concentration 3ppb, exposure time 6 weeks)
Molecular structure data
1. Molar refractive index: 35.82
2. Molar volume (cm3/mol): 104.0
3. Isotonic specific volume (90.2K ): 272.7
4. Surface tension (dyne/cm): 47.2
5. Polarizability (10-24cm3): 14.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 74.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain atomic stereocenterNumber of stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[12] Stable
2. Incompatible substances[13] Acid chlorides, acid anhydrides, strong oxidants
3. Conditions to avoid contact[14] Heating
4. Polymerization hazard[15] No polymerization
5. Decomposition products[16] Hydrogen bromide
Storage method
Storage Precautions[17] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from o-aminophenol by diazotization and bromination: (1) Diazotization Stir o-aminophenol and water evenly, add concentrated sulfuric acid and crushed ice and cool down to below 5℃. Slowly add sodium nitrite solution and keep the reaction temperature below 5°C to obtain diazonium salt. (2) Bromination Add cuprous bromide to hydrobromic acid to dissolve it, stir and add the heavy nitrogen salt, stir for 0.5h after adding, let it stand, steam distill until the distillate is oil-free, let it stand to separate the oil layer, and The oil is obtained by fractionation under reduced pressure. 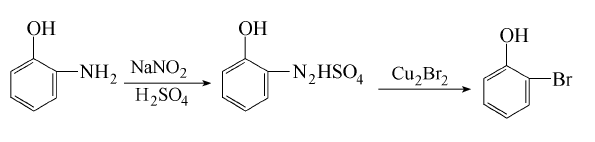
Purpose
1. Manufacturing disinfectants, used in organic synthesis, pharmaceutical intermediates, and solvents.
2. Used in organic synthesis. [18]
extended-reading:https://www.bdmaee.net/n-butyltintrichloridemin-95/extended-reading:https://www.newtopchem.com/archives/category/products/page/136extended-reading:https://www.bdmaee.net/desmorepid-so-catalyst-cas112-96-9-rhine-chemistry/extended-reading:https://www.bdmaee.net/polycat-17-pc-amine-ma-190-amine-balance-catalyst/extended-reading:https://www.cyclohexylamine.net/tetramethyl-13-diaminopropane-tmeda/extended-reading:https://www.cyclohexylamine.net/low-odor-amine-catalyst-bx405-low-odor-strong-gel-catalyst-bx405/extended-reading:https://www.newtopchem.com/archives/789extended-reading:https://www.newtopchem.com/archives/1604extended-reading:https://www.cyclohexylamine.net/rigid-foam-catalyst-semi-rigid-foam-catalyst/extended-reading:https://www.bdmaee.net/1-methylimidazole/