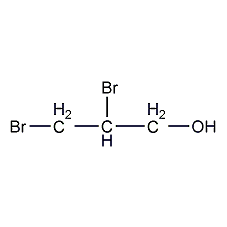
Structural formula
| Business number | 016H |
|---|---|
| Molecular formula | C9H9HgNaO2S |
| Molecular weight | 404.81 |
| label |
thimerosal, Thimerosal |
Numbering system
CAS number:54-64-8
MDL number:MFCD00013062
EINECS number:200-210-4
RTECS number:OV8400000
BRN number:8169555
PubChem number:24900257
Physical property data
1. Character:Colorless crystal or milky white crystalline powder. Stable in air but not in sunlight
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g /mL,AIR= 1): Undetermined
4. Melting point (ºC): Zhan232- 233℃
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC):250
9. Specific optical rotation (º):Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure (kPa,60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of water) partition coefficient: Undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility:1gThe product is dissolved in About1mlWater, about8mlEthanol. Almost insoluble in ether and benzene. 1%Aqueous solutionpHAbout6.7. The solution can be addedETDAAs a stabilizer.
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
1.Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 65.4
7. Heavy Number of atoms: 14
8. Surface charge: 0
9. Complexity: 180
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None
Storage method
None
Synthesis method
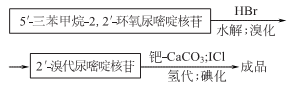
Prepared from the reaction of phosphorus thiol benzoic acid and ethylmercuric chloride
Purpose
is a disinfectant and antiseptic. It has antibacterial and antimycobacterial effects and is more effective than mercurochrome. It is weaker than mercury chloride, less toxic and irritating. For external use as a skin and mucous membrane disinfectant. (Used for skin wound disinfection, eye and nose mucosal inflammation, urethral lavage, and skin fungal infections.)
extended-reading:https://www.cyclohexylamine.net/dabco-dc1-delayed-catalyst-dabco-dc1/extended-reading:https://www.bdmaee.net/niax-c-248-tertiary-amine-catalyst-momentive/extended-reading:https://www.bdmaee.net/polyurethane-amine-catalyst-9727/extended-reading:https://www.bdmaee.net/tegoamin-pmdeta-catalyst-cas3030-47-5-degussa-ag/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/NNN-trimethyl-N-hydroxyethyl-bisaminoethyl-ether-CAS-83016-70-0-Jeffcat-ZF-10.pdfextended-reading:https://www.newtopchem.com/archives/43968extended-reading:https://www.cyclohexylamine.net/foam-amine-catalyst-strong-blowing-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/drier-butyl-tin-oxide-FASCAT-4101.pdfextended-reading:https://www.newtopchem.com/archives/39987extended-reading:https://www.newtopchem.com/archives/44867